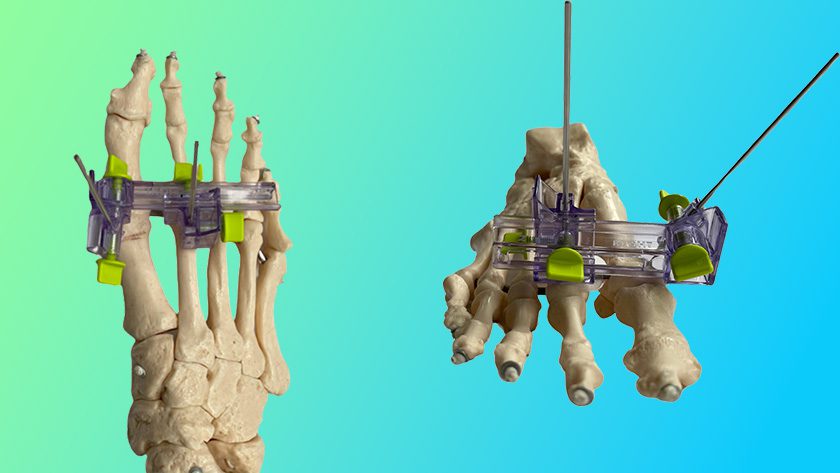
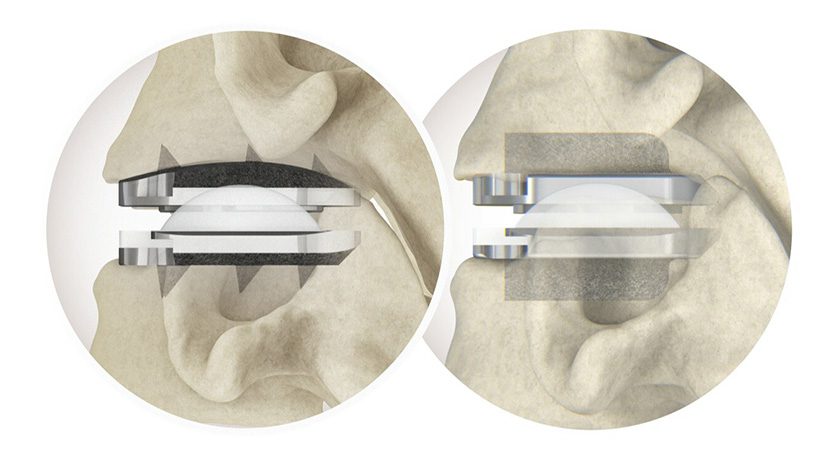
Stryker received FDA 510(k) clearance of the SAHARA® Lateral 3D Expandable Interbody System featuring Lamellar 3D Titanium Technology.
SAHARA Lateral is the first ever 3D-printed lateral expandable fusion device. Its passive expansion capabilities are designed to allow surgeons to achieve up to 30 degrees of sagittal spinal correction. The implant can be adjusted from a lateral approach intraoperatively, or can adjust passively in a staged posterior approach following excision of bone.
Lamellar 3D Titanium Technology is 3D printing method whereby implants are grown through selective application of a high-energy laser beam, creating complex internal geometries and a roughened surface architecture. The same technology was used to manufacture the MOJAVE posterior lumbar expandable interbody, launched by K2M in 2018 prior to the company’s acquisition by Stryker.
SAHARA Lateral complements the SAHARA Anterior Lumbar Expandable Stabilization System, a lordotic expandable device with integrated screw fixation introduced by K2M in 2017. It was K2M’s first expandable interbody device.
Stryker received FDA 510(k) clearance of the SAHARA® Lateral 3D Expandable Interbody System featuring Lamellar 3D Titanium Technology.
SAHARA Lateral is the first ever 3D-printed lateral expandable fusion device. Its passive expansion capabilities are designed to allow surgeons to achieve up to 30 degrees of sagittal spinal correction. The...
Stryker received FDA 510(k) clearance of the SAHARA® Lateral 3D Expandable Interbody System featuring Lamellar 3D Titanium Technology.
SAHARA Lateral is the first ever 3D-printed lateral expandable fusion device. Its passive expansion capabilities are designed to allow surgeons to achieve up to 30 degrees of sagittal spinal correction. The implant can be adjusted from a lateral approach intraoperatively, or can adjust passively in a staged posterior approach following excision of bone.
Lamellar 3D Titanium Technology is 3D printing method whereby implants are grown through selective application of a high-energy laser beam, creating complex internal geometries and a roughened surface architecture. The same technology was used to manufacture the MOJAVE posterior lumbar expandable interbody, launched by K2M in 2018 prior to the company’s acquisition by Stryker.
SAHARA Lateral complements the SAHARA Anterior Lumbar Expandable Stabilization System, a lordotic expandable device with integrated screw fixation introduced by K2M in 2017. It was K2M’s first expandable interbody device.


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.