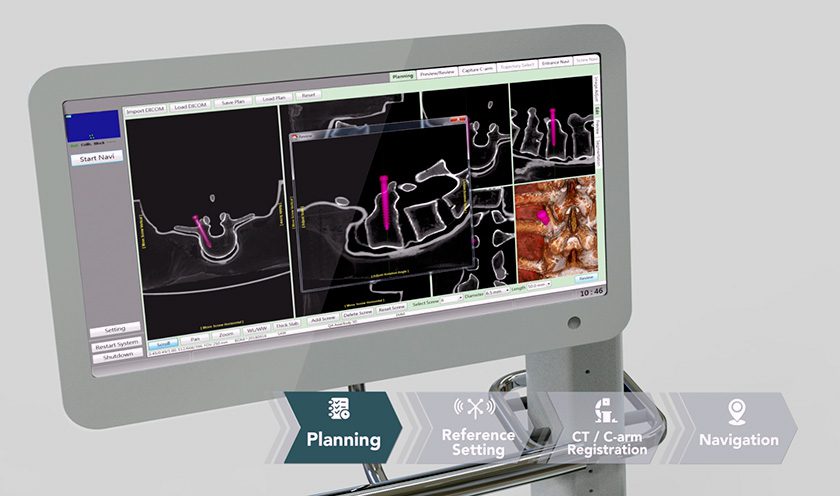
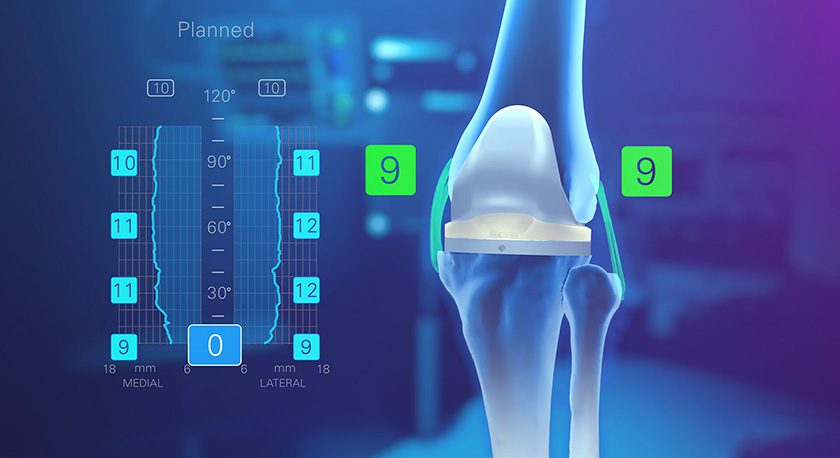
Precision Spine has received FDA 510(k) clearance for the AccuFit® Lateral Plate.
The titanium-based, low-profile AccuFit plate features four points of fixation and five sizes matched to the heights of ShurFit® LLIF Interbody cages. AccuFit is designed for use with the MD-Vue™ Lateral Access retractor, which Precision Spine debuted in 3Q16.
Sources: Precision Spine, Inc.; ORTHOWORLD Inc.
Precision Spine has received FDA 510(k) clearance for the AccuFit® Lateral Plate.
The titanium-based, low-profile AccuFit plate features four points of fixation and five sizes matched to the heights of ShurFit® LLIF Interbody cages. AccuFit is designed for use with the MD-Vue™ Lateral Access retractor, which Precision Spine debuted in 3Q16.
...
Precision Spine has received FDA 510(k) clearance for the AccuFit® Lateral Plate.
The titanium-based, low-profile AccuFit plate features four points of fixation and five sizes matched to the heights of ShurFit® LLIF Interbody cages. AccuFit is designed for use with the MD-Vue™ Lateral Access retractor, which Precision Spine debuted in 3Q16.
Sources: Precision Spine, Inc.; ORTHOWORLD Inc.


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.