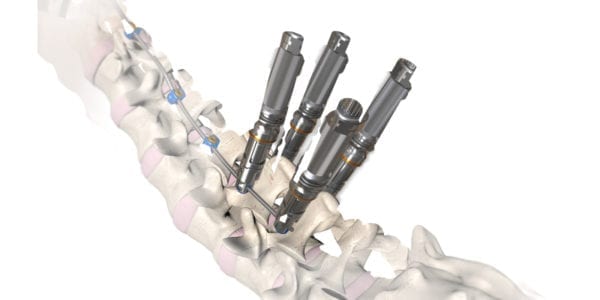






OrthoPediatrics was granted FDA 510(k) clearance to expand the indications for its RESPONSE™ Scoliosis System to include neuromuscular implants.
RESPONSE™ NeuroMuscular is based on the company’s RESPONSE™ Spine System, and will feature implants and instruments with specific attributes to simplify insertion and options to address extreme hyperlordosis.
Executive Vice President David Bailey said, “While we cannot provide our expected launch timeline given the current environment, we remain undeterred on our cause of improving the lives of children suffering from scoliosis. I’m pleased by the resolve of all our associates who continue to advance our business while working remotely, so that we can emerge from the COVID-19 crisis even stronger together.”
OrthoPediatrics was granted FDA 510(k) clearance to expand the indications for its RESPONSE™ Scoliosis System to include neuromuscular implants.
RESPONSE™ NeuroMuscular is based on the company’s RESPONSE™ Spine System, and will feature implants and instruments with specific attributes to simplify insertion and options to address extreme...
OrthoPediatrics was granted FDA 510(k) clearance to expand the indications for its RESPONSE™ Scoliosis System to include neuromuscular implants.
RESPONSE™ NeuroMuscular is based on the company’s RESPONSE™ Spine System, and will feature implants and instruments with specific attributes to simplify insertion and options to address extreme hyperlordosis.
Executive Vice President David Bailey said, “While we cannot provide our expected launch timeline given the current environment, we remain undeterred on our cause of improving the lives of children suffering from scoliosis. I’m pleased by the resolve of all our associates who continue to advance our business while working remotely, so that we can emerge from the COVID-19 crisis even stronger together.”


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







