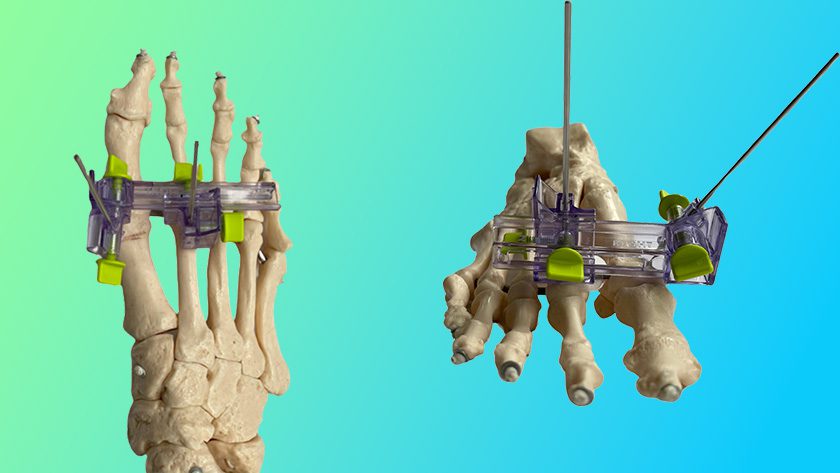
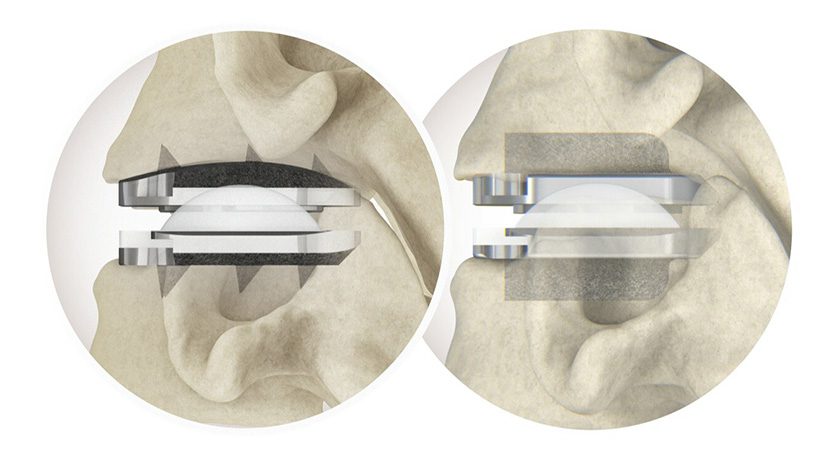
NuVasive received FDA 510(k) clearance and CE Mark approval to market Precice® Bone Transport, an all-internal treatment for segmental bone loss in the tibia and femur resulting from trauma or infection. The system is slated for 3Q19 launch in the U.S. and Europe.
The system is part of the NuVasive Specialized Orthopedics (NSO) portfolio of magnetically adjustable limb-lengthening products. It includes an implantable, magnetic stainless steel intramedullary nail with a dual slot designed to support the transport of an intercalary bone segment to facilitate regeneration in bone defects. An external remote controller moves the bone segment up to 10 centimeters.
Other NSO products include Precice Stryde™, an intramedullary limb-lengthening system cleared by FDA and CE marked in 2018 for limb lengthening of the femur and tibia, and the MAGEC® system to treat severe spinal deformity.
Sources: NuVasive; ORTHOWORLD Inc.
NuVasive received FDA 510(k) clearance and CE Mark approval to market Precice® Bone Transport, an all-internal treatment for segmental bone loss in the tibia and femur resulting from trauma or infection. The system is slated for 3Q19 launch in the U.S. and Europe.
The system is part of the NuVasive Specialized Orthopedics (NSO) portfolio of...
NuVasive received FDA 510(k) clearance and CE Mark approval to market Precice® Bone Transport, an all-internal treatment for segmental bone loss in the tibia and femur resulting from trauma or infection. The system is slated for 3Q19 launch in the U.S. and Europe.
The system is part of the NuVasive Specialized Orthopedics (NSO) portfolio of magnetically adjustable limb-lengthening products. It includes an implantable, magnetic stainless steel intramedullary nail with a dual slot designed to support the transport of an intercalary bone segment to facilitate regeneration in bone defects. An external remote controller moves the bone segment up to 10 centimeters.
Other NSO products include Precice Stryde™, an intramedullary limb-lengthening system cleared by FDA and CE marked in 2018 for limb lengthening of the femur and tibia, and the MAGEC® system to treat severe spinal deformity.
Sources: NuVasive; ORTHOWORLD Inc.


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.