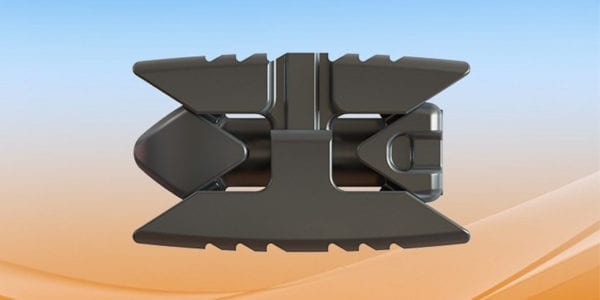






Life Spine received an additional 510(k) clearance from FDA to market the Lateral PROLIFT Expandable System. Further, the company plans to launch 20 new products in 2020, including six Micro Invasive Expandables.
Life Spine’s expandable portfolio includes:
- Lateral PROLIFT Expandable System
- 10mm and 12mm PROLIFT Expandable Systems for TLIF and PLIF approaches
- AILERON® TRX Expandable Interspinous Spacer
- LONGBOW® Expandable Lateral Spacer System
- LONGBOW Expandable TLIF Spacer System
- TiBOW™ Expandable Spacer System with horizontal expansion for a TLIF approach
“This year we will add some pivotal new technologies to our already robust expandable portfolio bringing our total number of expandable devices to 13,” said Rich Mueller, Chief Operating Officer for Life Spine. “Investing in expandable technologies is important to us because expandables are designed to allow for a truly Micro Invasive procedure by going in small and opening to match each patient’s unique anatomy. Our dedication to offering expandable solutions dates back to 2006 when we filed our first patent around expandable technology and now we aim to file one patent each month.”
Life Spine received an additional 510(k) clearance from FDA to market the Lateral PROLIFT Expandable System. Further, the company plans to launch 20 new products in 2020, including six Micro Invasive Expandables.
Life Spine’s expandable portfolio includes:
Lateral PROLIFT Expandable System
10mm and 12mm PROLIFT Expandable Systems...
Life Spine received an additional 510(k) clearance from FDA to market the Lateral PROLIFT Expandable System. Further, the company plans to launch 20 new products in 2020, including six Micro Invasive Expandables.
Life Spine’s expandable portfolio includes:
- Lateral PROLIFT Expandable System
- 10mm and 12mm PROLIFT Expandable Systems for TLIF and PLIF approaches
- AILERON® TRX Expandable Interspinous Spacer
- LONGBOW® Expandable Lateral Spacer System
- LONGBOW Expandable TLIF Spacer System
- TiBOW™ Expandable Spacer System with horizontal expansion for a TLIF approach
“This year we will add some pivotal new technologies to our already robust expandable portfolio bringing our total number of expandable devices to 13,” said Rich Mueller, Chief Operating Officer for Life Spine. “Investing in expandable technologies is important to us because expandables are designed to allow for a truly Micro Invasive procedure by going in small and opening to match each patient’s unique anatomy. Our dedication to offering expandable solutions dates back to 2006 when we filed our first patent around expandable technology and now we aim to file one patent each month.”


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







