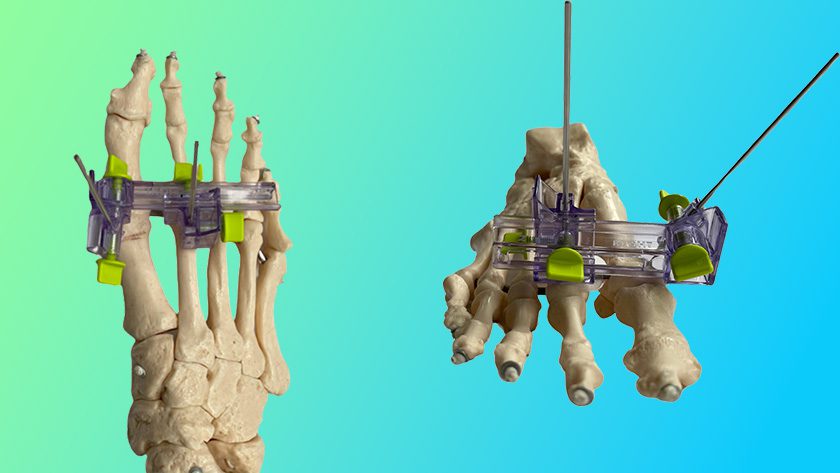
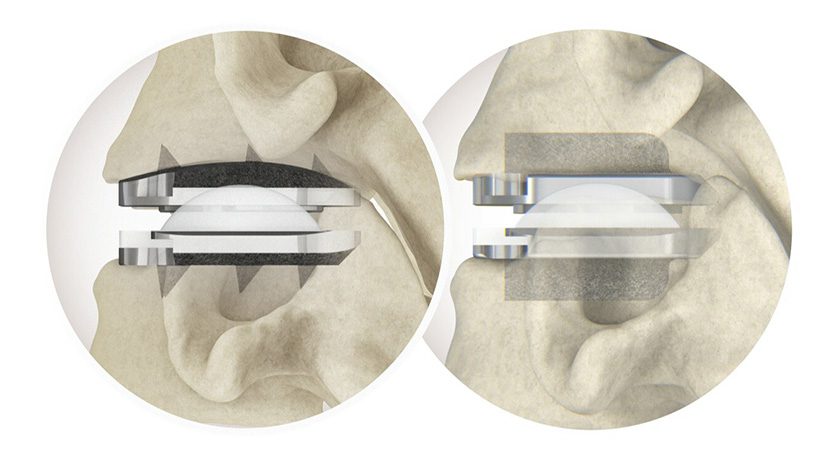
Gramercy Extremity Orthopedics (GEO) received FDA 510(k) clearance to market the GEO™ Bone Screw, indicated for fractures, osteotomies, arthrodesis, osteochondritis and tendon reattachment. This is the company’s first 510(k) clearance.
The GEO system comprises an array of low-profile titanium screw lengths, diameters, thread lengths and fully-threaded options. Screws are double sterile packaged, self-drilling, self-tapping, reverse-cutting with variable length short and long threads and a hexalobe head to provide additional stability and torque transfer with less potential for head stripping.
Instruments are also double sterile packaged in single use kits.
GEO was formed with the objective to lower real operating costs by creating efficiencies and controls throughout delivery and consumption of orthopaedic implants.
Gramercy Extremity Orthopedics (GEO) received FDA 510(k) clearance to market the GEO™ Bone Screw, indicated for fractures, osteotomies, arthrodesis, osteochondritis and tendon reattachment. This is the company's first 510(k) clearance.
The GEO system comprises an array of low-profile titanium screw lengths, diameters, thread lengths and...
Gramercy Extremity Orthopedics (GEO) received FDA 510(k) clearance to market the GEO™ Bone Screw, indicated for fractures, osteotomies, arthrodesis, osteochondritis and tendon reattachment. This is the company’s first 510(k) clearance.
The GEO system comprises an array of low-profile titanium screw lengths, diameters, thread lengths and fully-threaded options. Screws are double sterile packaged, self-drilling, self-tapping, reverse-cutting with variable length short and long threads and a hexalobe head to provide additional stability and torque transfer with less potential for head stripping.
Instruments are also double sterile packaged in single use kits.
GEO was formed with the objective to lower real operating costs by creating efficiencies and controls throughout delivery and consumption of orthopaedic implants.
Source: Gramercy Extremity Orthopedics LLC


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.