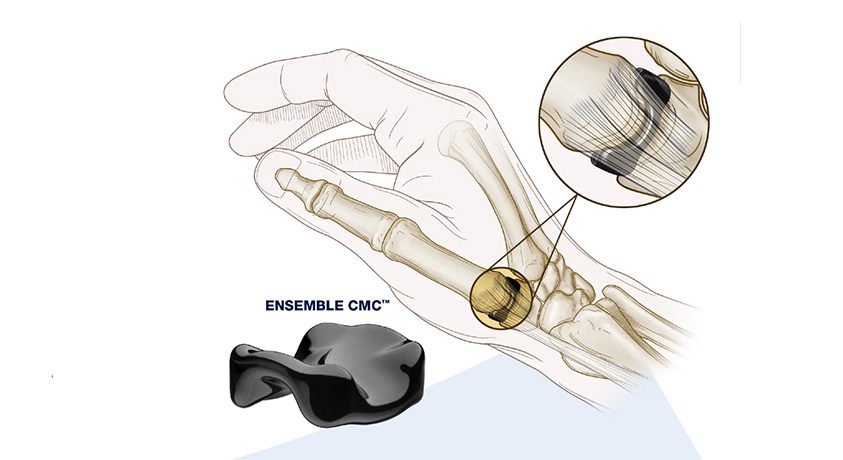
On 4/20/16, FDA’s Orthopaedic and Rehabilitation Devices Panel will meet regarding the PMA for Cartiva’s Synthetic Cartilage Implant (SCI), an organic polymer-based biomaterial designed to mimic biologic cartilage.
SCI is to be indicated for treatment of degenerative and post-traumatic arthritis in the first metatarsophalangeal joint, in the presence of good bone stock and with the following clinical conditions: hallux valgus or hallux limitus, hallux rigidus and an unstable or painful metatarsophalangeal joint.
Source: GPO.gov
In 3Q15, Cartiva announced results from a Phase III randomized, controlled trial of SCI for the treatment of OA of the first metatarsophalangeal joint. Results indicated durable pain reduction and functional improvement similar to fusion, but with a statistically significant improvement in range of motion and a faster return to function.
On 4/20/16, FDA's Orthopaedic and Rehabilitation Devices Panel will meet regarding the PMA for Cartiva's Synthetic Cartilage Implant (SCI), an organic polymer-based biomaterial designed to mimic biologic cartilage.
SCI is to be indicated for treatment of degenerative and post-traumatic arthritis in the first metatarsophalangeal joint, in the...
On 4/20/16, FDA’s Orthopaedic and Rehabilitation Devices Panel will meet regarding the PMA for Cartiva’s Synthetic Cartilage Implant (SCI), an organic polymer-based biomaterial designed to mimic biologic cartilage.
SCI is to be indicated for treatment of degenerative and post-traumatic arthritis in the first metatarsophalangeal joint, in the presence of good bone stock and with the following clinical conditions: hallux valgus or hallux limitus, hallux rigidus and an unstable or painful metatarsophalangeal joint.
Source: GPO.gov
In 3Q15, Cartiva announced results from a Phase III randomized, controlled trial of SCI for the treatment of OA of the first metatarsophalangeal joint. Results indicated durable pain reduction and functional improvement similar to fusion, but with a statistically significant improvement in range of motion and a faster return to function.


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.