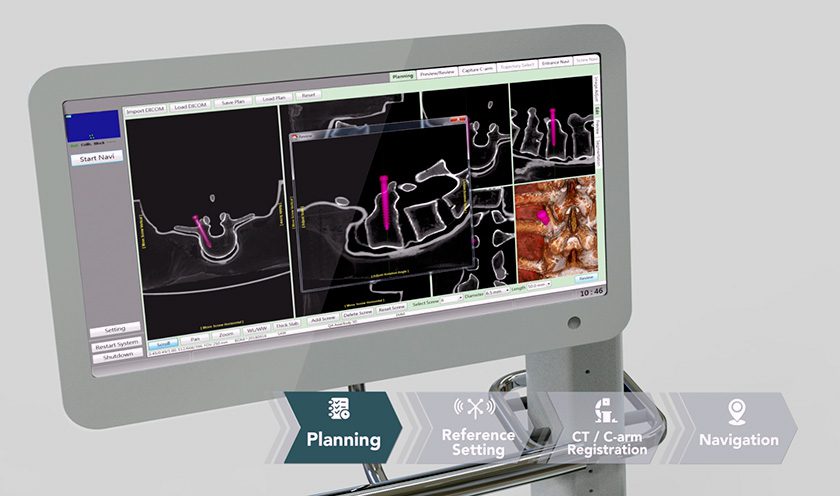
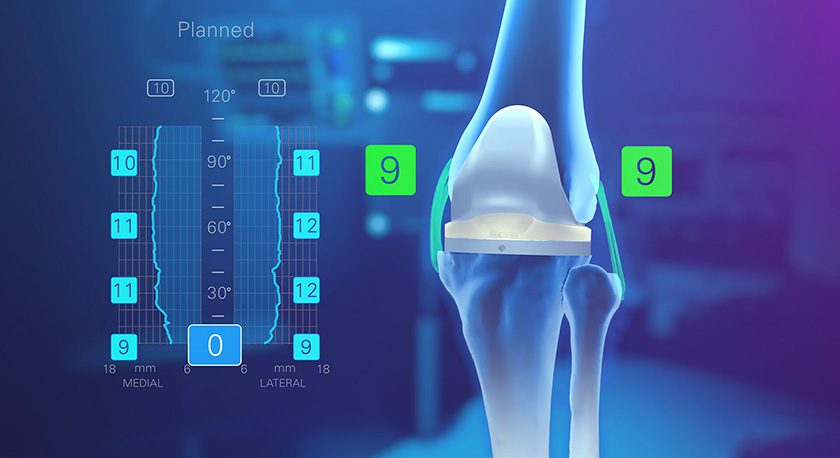
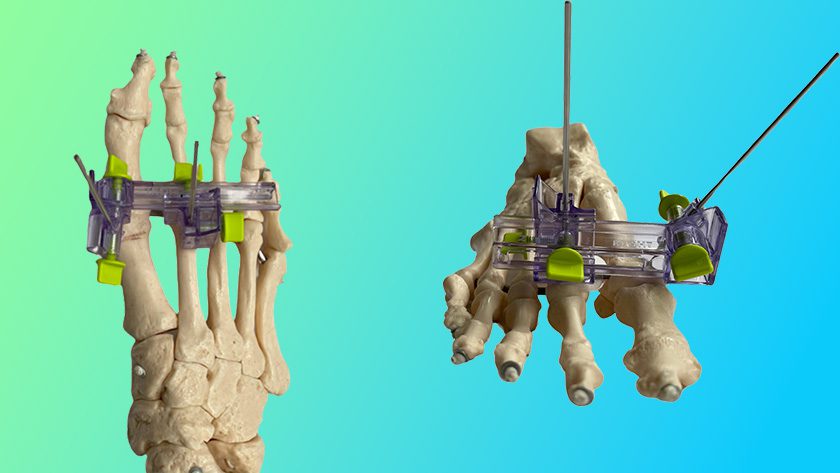
Medicrea received FDA 510(k) clearances for its PASS® XS posterior fixation and LigaPASS® XS band connector components, designed to address pediatric spinal deformities in small stature patients.
The extra-small XS extensions of PASS and LigaPASS technology will allow surgeons to treat pediatric patients using ~40% less implant volume in each surgery. Like the adult-sized implants, these versions can be used with UNiD™ Lab patient-specific digital surgical planning and analytical services.
First U.S. surgery with PASS XS and LigaPASS XS are expected in mid-4Q16.
Source: Medicrea
Medicrea received FDA 510(k) clearances for its PASS® XS posterior fixation and LigaPASS® XS band connector components, designed to address pediatric spinal deformities in small stature patients.
The extra-small XS extensions of PASS and LigaPASS technology will allow surgeons to treat pediatric patients using ~40% less implant volume in each...
Medicrea received FDA 510(k) clearances for its PASS® XS posterior fixation and LigaPASS® XS band connector components, designed to address pediatric spinal deformities in small stature patients.
The extra-small XS extensions of PASS and LigaPASS technology will allow surgeons to treat pediatric patients using ~40% less implant volume in each surgery. Like the adult-sized implants, these versions can be used with UNiD™ Lab patient-specific digital surgical planning and analytical services.
First U.S. surgery with PASS XS and LigaPASS XS are expected in mid-4Q16.
Source: Medicrea


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.