





CarboFix received FDA 510(k) clearance to market CarboClear® Carbon Fiber fenestrated pedicle screws to treat advanced-stage spinal tumors. Product launch is underway.
The system is used with Teknimed’s High V+ high viscosity radiopaque PMMA bone cement to enhance fixation in compromised bone.
CarboClear is reportedly the only pedicle screw made entirely of carbon fibers, which benefit oncological patients with high fatigue strength to support an impaired healing process and no backscattering, which allows the use of radiation therapy without harming the healthy surrounding tissue. Unlike titanium material, no artifacts appear under MRI.
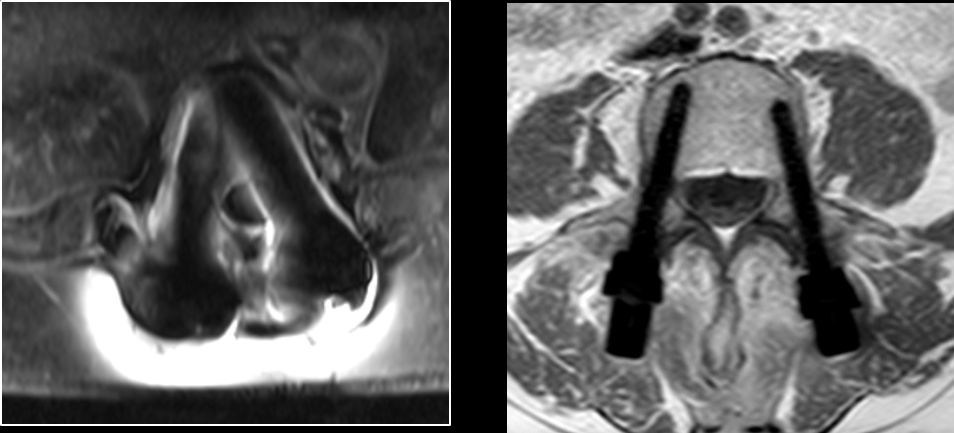   |
MRI images: Titanium pedicle screws (left) and CarboClear pedicle screws (right) |
Source: CarboFix
CarboFix received FDA 510(k) clearance to market CarboClear® Carbon Fiber fenestrated pedicle screws to treat advanced-stage spinal tumors. Product launch is underway.
The system is used with Teknimed's High V+ high viscosity radiopaque PMMA bone cement to enhance fixation in compromised bone.
CarboClear is reportedly the only pedicle screw...
CarboFix received FDA 510(k) clearance to market CarboClear® Carbon Fiber fenestrated pedicle screws to treat advanced-stage spinal tumors. Product launch is underway.
The system is used with Teknimed’s High V+ high viscosity radiopaque PMMA bone cement to enhance fixation in compromised bone.
CarboClear is reportedly the only pedicle screw made entirely of carbon fibers, which benefit oncological patients with high fatigue strength to support an impaired healing process and no backscattering, which allows the use of radiation therapy without harming the healthy surrounding tissue. Unlike titanium material, no artifacts appear under MRI.
   |
MRI images: Titanium pedicle screws (left) and CarboClear pedicle screws (right) |
Source: CarboFix


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







