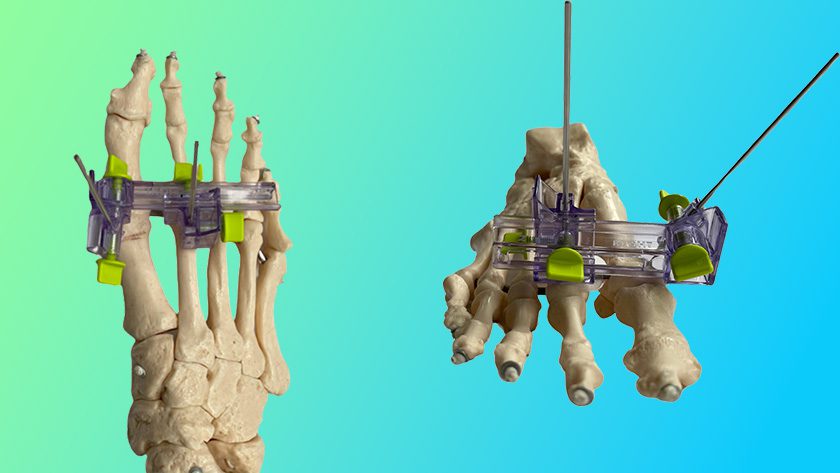
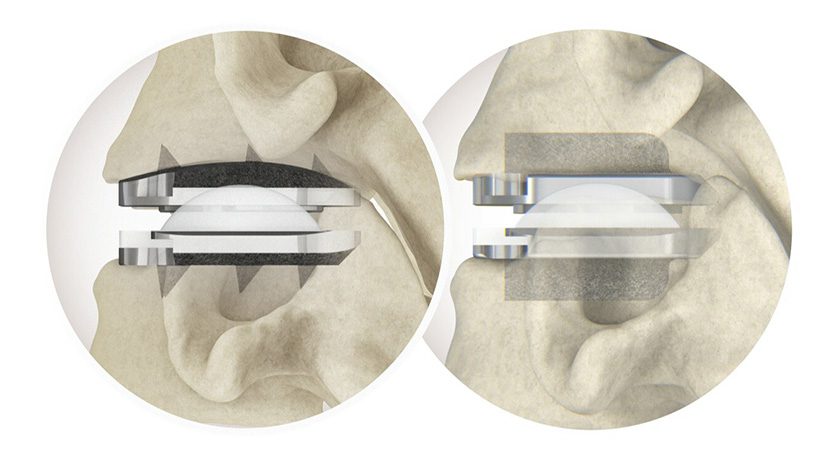
Amplitude Surgical received FDA 510(k) clearance to market the Anatomic® implant for the treatment of degenerative knee disease.
The company filed for 510(k) clearance of the device in 2Q16, at which time it had been used in >17,000 procedures in ex-U.S. launch. To date, that number has increased to >20,000 procedures.
The company will now proceed to launch its U.S. subsidiary, Amplitude Orthopedics Corp. Amplitude claims close to 15% of the French market for knee implants, ranking second in the country behind Zimmer Biomet, and ahead of DePuy, Stryker and Smith & Nephew in the region.
Sources: Amplitude Surgical; ORTHOWORLD Inc.
Amplitude Surgical received FDA 510(k) clearance to market the Anatomic® implant for the treatment of degenerative knee disease.
The company filed for 510(k) clearance of the device in 2Q16, at which time it had been used in >17,000 procedures in ex-U.S. launch. To date, that number has increased to >20,000 procedures.
The company...
Amplitude Surgical received FDA 510(k) clearance to market the Anatomic® implant for the treatment of degenerative knee disease.
The company filed for 510(k) clearance of the device in 2Q16, at which time it had been used in >17,000 procedures in ex-U.S. launch. To date, that number has increased to >20,000 procedures.
The company will now proceed to launch its U.S. subsidiary, Amplitude Orthopedics Corp. Amplitude claims close to 15% of the French market for knee implants, ranking second in the country behind Zimmer Biomet, and ahead of DePuy, Stryker and Smith & Nephew in the region.
Sources: Amplitude Surgical; ORTHOWORLD Inc.


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.