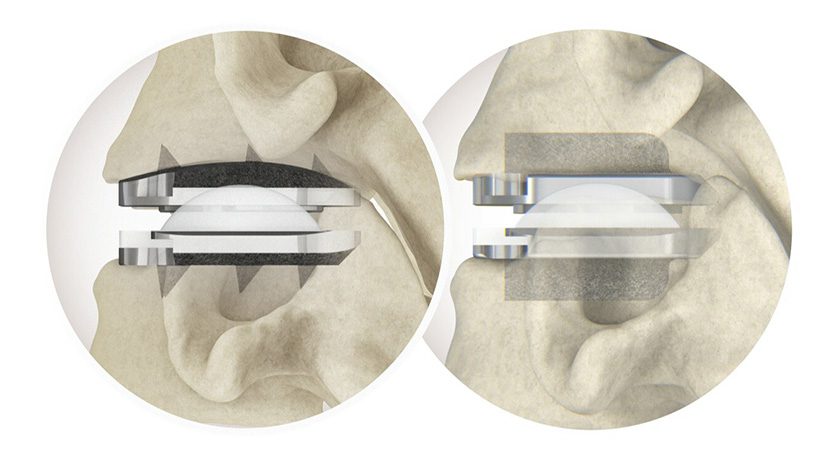
Additive Orthopaedics received FDA 510(k) clearance for its 3D-printed intramedullary Bunion Correction System.
This is the company’s fourth 510(k) clearance employing the additive manufacturing process and its sixth complete product line, which includes implants, biologics and customs for limb salvage and complex revision in the foot and ankle. Since the first product launch in late 2016, >400 Additive Orthopaedics devices have been implanted.
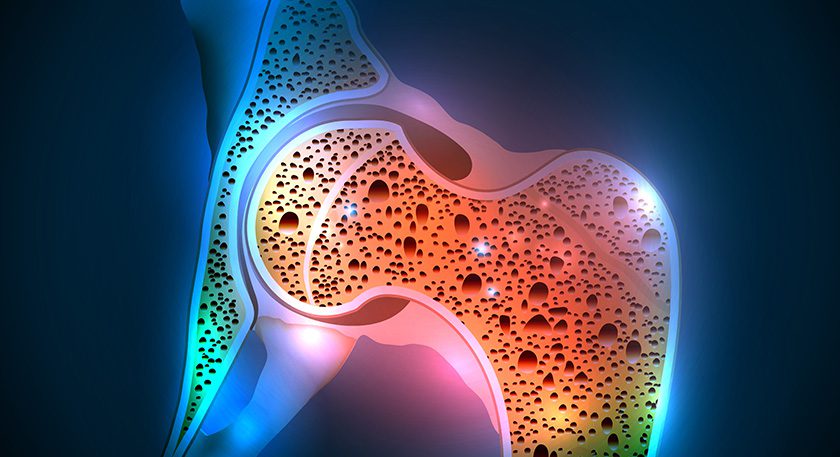
In 1Q17, the company launched a multi-center clinical study to measure bone in-growth into 3D-printed bone segments. Leadership has noted that honeycomb lattice structures have demonstrated improvements in post-op success and potential bone in-growth vs. those achieved with current allograft wedge options.
Sources: Additive Orthopaedics, LLC; ORTHOWORLD Inc.
Additive Orthopaedics received FDA 510(k) clearance for its 3D-printed intramedullary Bunion Correction System.
This is the company's fourth 510(k) clearance employing the additive manufacturing process and its sixth complete product line, which includes implants, biologics and customs for limb salvage and complex revision in the...
Additive Orthopaedics received FDA 510(k) clearance for its 3D-printed intramedullary Bunion Correction System.
This is the company’s fourth 510(k) clearance employing the additive manufacturing process and its sixth complete product line, which includes implants, biologics and customs for limb salvage and complex revision in the foot and ankle. Since the first product launch in late 2016, >400 Additive Orthopaedics devices have been implanted.
In 1Q17, the company launched a multi-center clinical study to measure bone in-growth into 3D-printed bone segments. Leadership has noted that honeycomb lattice structures have demonstrated improvements in post-op success and potential bone in-growth vs. those achieved with current allograft wedge options.
Sources: Additive Orthopaedics, LLC; ORTHOWORLD Inc.


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.