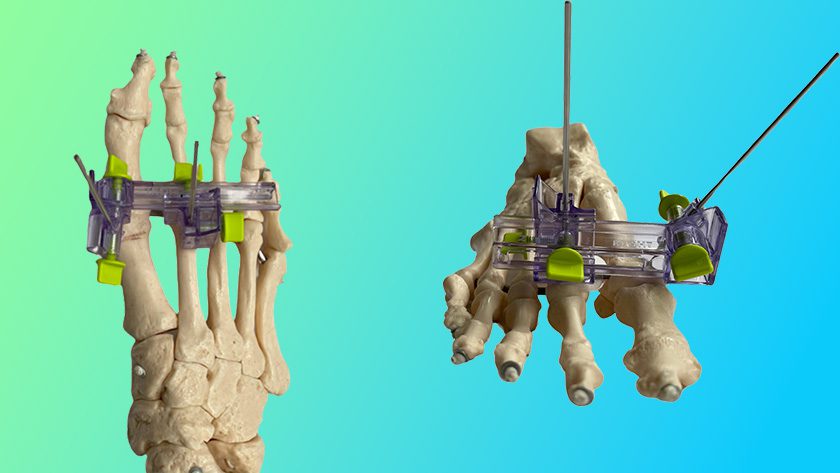
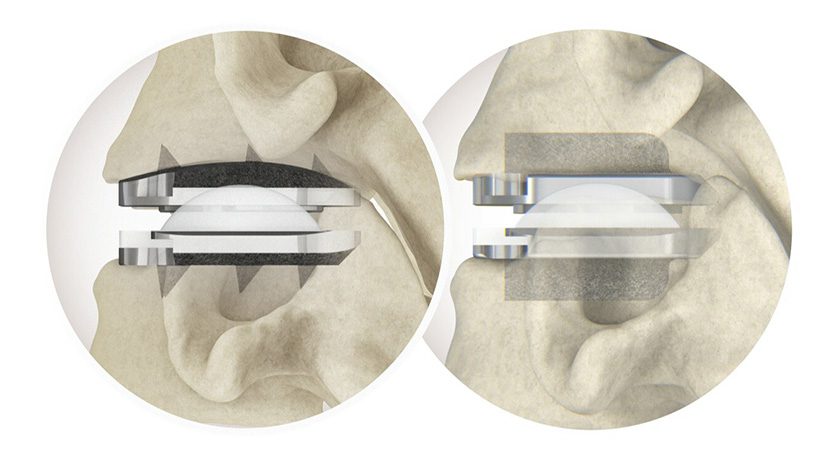
Zimmer Biomet (ZBH) received FDA 510(k) clearance for compatibility of the Nexel™ Total Elbow with the Comprehensive® Segmental Revision System (SRS). This marks the 1st submission by ZBH to establish compatibility of two separate ZBH implant systems.
The distal component of Biomet’s Comprehensive SRS humeral system, when combined with Zimmer’s Nexel Total Elbow, is designed for elbow joint replacement, while the remaining components support proximal or total humeral reconstruction when used with the glenoid component of the Comprehensive Shoulder.
Further, the ability of Comprehensive SRS and Nexel to interact with Regenerex™ Tissue Attachment Augments addresses concerns regarding graft availability and resorption.
Source: Zimmer Biomet, Inc.
Zimmer Biomet (ZBH) received FDA 510(k) clearance for compatibility of the Nexel™ Total Elbow with the Comprehensive® Segmental Revision System (SRS). This marks the 1st submission by ZBH to establish compatibility of two separate ZBH implant systems.
The distal component of Biomet's Comprehensive SRS humeral system, when combined with...
Zimmer Biomet (ZBH) received FDA 510(k) clearance for compatibility of the Nexel™ Total Elbow with the Comprehensive® Segmental Revision System (SRS). This marks the 1st submission by ZBH to establish compatibility of two separate ZBH implant systems.
The distal component of Biomet’s Comprehensive SRS humeral system, when combined with Zimmer’s Nexel Total Elbow, is designed for elbow joint replacement, while the remaining components support proximal or total humeral reconstruction when used with the glenoid component of the Comprehensive Shoulder.
Further, the ability of Comprehensive SRS and Nexel to interact with Regenerex™ Tissue Attachment Augments addresses concerns regarding graft availability and resorption.
Source: Zimmer Biomet, Inc.


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.